Biofilm and Greenhouses
Biofilm and greenhouses come hand in hand.
Canadian greenhouse agriculture represents a rapidly expanding sector within the controlled environment agriculture industry, with significant implications for food security and sustainable production (Morales Moreira & Haney, 2024; Bambara et al., 2021). Biofilm formation in these systems presents both challenges and opportunities for optimizing crop health, system efficiency, and microbial management (Tham et al., 2024; Legein et al., 2022). Understanding the complex microbial ecology of biofilms in hydroponic and controlled environment systems is crucial for developing effective management strategies that can enhance beneficial microbial interactions while preventing pathogen establishment (Thomas et al., 2025; Thomas et al., 2024).
Biofilms: Definition and Fundamental Characteristics
Biofilms are structured communities of microorganisms embedded within a self-produced matrix of extracellular polymeric substances (EPS) that adhere to surfaces (Yin et al., 2019; Lewandowski & Beyenal, 2009). These complex three-dimensional structures represent a predominant mode of microbial life, with bacteria transitioning from planktonic (free-floating) to sessile (surface-attached) lifestyles (Beitelshees et al., 2018; Wong et al., 2023). The biofilm matrix, composed primarily of polysaccharides, proteins, lipids, and nucleic acids, provides protection against environmental stresses including antimicrobial compounds, pH fluctuations, and nutrient limitation (Yin et al., 2019; Wang et al., 2020).
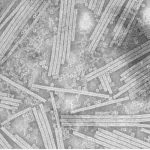
Biofilm formation and dispersal stages, including attachment, maturation, and dispersion (Nature). Image by Rumbaugh& Sauer (2020).
The development of biofilms follows a multi-stage process involving an initial reversible adhesion, irreversible attachment, maturation, and eventual dispersion (Lobo-Cabrera et al., 2024; Beitelshees et al., 2018). During the initial adhesion phase, planktonic bacteria approach surfaces through Brownian motion, sedimentation, or convective transport, followed by weak physicochemical interactions (Chepkwony & Brun, 2021).. Irreversible attachment occurs through specific adhesin–receptor interactions and the production of adhesive polymers, leading to microcolonies (Billaud et al., 2022; Tavano et al., 2018). The maturation phase involves extensive EPS production, formation of complex three-dimensional architectures with water channels, and the development of chemical gradients within the biofilm structure (Lobo-Cabrera et al., 2024; Culioli et al., 2024).
Simply put, microbes will float through various means, stick to a surface, and grow into a complex colony with water channels and chemical layers.

We have scanned over 500+ different types of organisms
You can check out the list by clicking the button.
Biofilms in Indoor Agriculture and Greenhouse Systems
Mechanisms of Biofilm Formation in Greenhouses
Greenhouse and hydroponic biofilms begin when free-floating (planktonic) microbes adhere to abiotic surfaces such as PVC and stainless-steel piping, pump housings, and soilless media via extracellular polymeric substances (EPS), forming a slimy matrix that further captures nutrients and cells. Hydroponic setups, with their continuous flow of nutrient-rich solution, create ideal conditions for microbial adhesion and proliferation on equipment and plant root surfaces, as documented in sanitization studies of Singapore NFT facilities (Tham et al., 2024).
Once established, biofilms protect resident bacteria and fungi from environmental stresses and sanitizers, making them notoriously difficult to eradicate without rigorous cleaning protocols (Brackman, 2019).
Greenhouse Operational Impacts and Risks of Biofilms
Uncontrolled biofilm accumulation narrows irrigation channels and drippers, reducing flow rates and causing uneven nutrient delivery that can stunt crop growth and require costly downtime for maintenance (Chowdhury et al., 2024). Moreover, biofilms serve as reservoirs for plant and human pathogens, such as Pythium, Phytophthora, and even Salmonella spp., increasing disease incidence in leafy greens and other high-value greenhouse crops (National Institute of Food and Agriculture, 2023). Beyond disease, persistent biofilms elevate energy use (via increased pumping pressure), degrade equipment lifespan, and inflate operational costs, underscoring the economic importance of effective biofilm management (Dramm Corporation, 2012).

Biofilm Challenges in Canadian Greenhouses
In Canada, “most greenhouse vegetable production … is done using a hydroponic system” that recirculates nutrient solutions to maximize water efficiency and crop consistency (Agriculture and Agri-Food Canada, 2021). Ontario growers report that recirculated solutions frequently accumulate salts, microbial contaminants, and biofilm in the distribution network and root zone, necessitating ongoing solution monitoring and adjustment of EC/pH to mitigate buildup (Al-Daoud & McCreary, 2020; West et al., 2018).
Factors Influencing Biofilm Microbial Ecology in Greenhouses
Temperature Effects
Temperature represents a critical environmental parameter influencing biofilm formation in Canadian greenhouse systems (Ansari et al., 2022; Silva et al., 2022; Yin et al., 2019; Velez Correa et al., 2023). Research on bacterial biofilm formation demonstrates that optimal temperatures range from 25 °C to 37 °C, with significant reductions in biofilm density occurring at suboptimal temperatures (Ansari et al., 2022; Silva et al., 2022). Studies show biofilm density decreasing by 30 % at 25 °C and over 50 % at 45 °C compared to optimal conditions (Ansari et al., 2022). Additionally, modeling of pH–temperature interactions in Vibrio spp. has highlighted severe inhibition of planktonic growth and biofilm formation below ~20 °C and enhancement near 30 °C (Velez Correa et al., 2023).
In Canadian greenhouse operations, maintaining consistent temperatures within this optimal range requires significant energy input, particularly during winter months when external temperatures can drop well below freezing (Léveillée-Dallaire et al., 2023; Strack et al., 2024; Dramm Corporation, 2012). Biofilms also confer protection against thermal stresses, acting as microbial “protective clothing” under transient heat or cold shocks (Yin et al., 2019; Dramm Corporation, 2012). Field surveys of greenhouse drip irrigation systems have further revealed biofilm communities dominated by thermophilic, spore-forming taxa—underscoring their resilience to high-temperature regimes and potential impact on system clogging (Sánchez et al., 2014).
Continuous temperature monitoring is handled via on-site greenhouse climate control systems and complemented with lab assays.
- Engaging SGS Crop Science’s team or climate-optimization consulting to help integrate, interpret, and act on the temperature data.
- Installing environmental sensors (e.g. thermocouples or dataloggers) in your greenhouse.
pH and Chemical Environment
The pH of nutrient solutions significantly influences biofilm formation, with optimal ranges typically between 6.0 and 8.0 (Silva et al., 2022; Velez Correa et al., 2023). Deviations from neutral pH conditions substantially impact biofilm development, with acidic conditions (pH 5.0) and alkaline conditions (pH 9.0) reducing biofilm formation to 40 % and 35 % of peak density, respectively (Ansari et al., 2022). Canadian greenhouse operations utilizing standard hydroponic nutrient solutions, such as Hoagland’s solution, must carefully monitor and maintain pH levels to optimize beneficial biofilm formation while preventing pathogenic species establishment (Lekshmi & Sreekumar, 2025; Wdowikowska et al., 2023).
Ionic strength and salinity levels present additional complexity in biofilm management, with different bacterial species exhibiting varying responses to salt concentrations (Silva et al., 2022; Chepkwony & Brun, 2021). Research demonstrates that optimal biofilm formation occurs at 4 % NaCl for some species, while others require lower concentrations (Silva et al., 2022). Canadian greenhouse operations must consider water quality parameters, particularly in regions where source water contains elevated mineral content (Logozzo et al., 2025).
To monitor nutrient-solution pH, ionic strength and related chemical parameters, use:
- SGS Water & Nutrient Solution Analysis – Complete Solution
- Measures pH, EC, nitrate-N, P, K, Ca, Na, chloride, sulphate, Zn, Mn, Cu, Fe, B, Si, Mo, NH₄-N, bicarbonate.
- SGS Water & Nutrient Solution Analysis – Complete Water
- Similar scope plus total hardness and total dissolved solids.
These packages let you track how solution pH and salinity (ionic strength) shift over time, which is critical given that deviations outside pH 6–8 can strongly inhibit or alter biofilm formation.
Nutrient Availability and Flow Dynamics
Nutrient concentration and composition play fundamental roles in biofilm development, with carbon and nitrogen sources being particularly critical (Lobo-Cabrera et al., 2024; Wdowikowska et al., 2023). Studies on cucumber cultivation using recycled wastewater demonstrated that nutrient-rich environments can support robust biofilm formation, though careful supplementation is required to maintain plant health (Wdowikowska et al., 2023). The balance between providing adequate nutrients for plant growth while minimizing conditions that promote pathogenic biofilm formation represents a key challenge in hydroponic system management (Thomas et al., 2025; Tham et al., 2024).

Greenhouse irrigation pipe clogging has been linked to total suspended solids thresholds beyond which dripper uniformity degrades, and both pipe material and irrigation scheduling influence biofilm accumulation (Cabrera Garcia, 2019). Integrated biocontrol and sanitation approaches—such as beneficial Pseudomonas chlororaphis inoculation combined with UV-C irradiation—have been shown to suppress biofilm formation while enhancing crop yield in hydroponic lettuce systems (Lee et al., 2015).
Flow rate and shear stress significantly impact biofilm formation and structure (Rajcoomar et al., 2024; Culioli et al., 2024). Research on marine biofilm communities demonstrates that static conditions promote maximum biofilm formation, while increasing shear stress leads to decreased biomass but increased EPS production as an adaptive response (Culioli et al., 2024). In Canadian NFT systems, optimizing flow rates requires balancing adequate nutrient delivery to plants with biofilm management considerations (Tham et al., 2024).
Too quantify nutrient concentrations and understand how flow regimes influence biofilm growth:
- SGS Water & Nutrient Solution Analysis (Complete Solution or Complete Water) for major and micronutrient levels in recirculated solutions
- SGS Media Analysis (Basic or Complete) to assess nutrient content and EC in soilless media
- SGS Plant Tissue Analysis (Basic or Complete) to monitor plant nutrient uptake, which reflects actual delivery under your flow conditions
Together, these allow you to balance nutritional needs for crops while minimizing conditions that favor excessive biofilm buildup.
Microbial Community Interactions
The composition and diversity of microbial communities significantly influence biofilm formation and stability (Wang et al., 2020; Liu et al., 2020). Research on hybrid membrane bioreactors demonstrates that higher species richness and diversity correlate with reduced problematic biofilm formation, suggesting that maintaining balanced microbial communities can serve as a biocontrol strategy (Liu et al., 2020). Canadian greenhouse operations can potentially leverage plant genetic regulation of microbiome composition to optimize beneficial biofilm formation while suppressing pathogenic species (Morales Moreira & Haney, 2024).

Emerging contaminants such as microplastics have also been shown to act as novel substrates for biofilm development, with plastic type influencing EPS composition and biomass accumulation—factors that may affect nutrient cycling and pollutant transport in greenhouse soils (Chen et al., 2022). Studies on rhizobacterial species richness demonstrate that increased microbial diversity enhances plant growth and soil nutrient synergism (Sahib et al., 2020). This research suggests that promoting diverse microbial communities in hydroponic root zones could optimize both plant health and biofilm management (Sahib et al., 2020).
For profiling and tracking the composition and dynamics of biofilm communities:
- HH Pathogen+ Full Scan (500+ bacteria, fungi, fungal-like organisms by amplicon sequencing) for comprehensive community snapshots and temporal trend-tracking
- HH Pathogen+ Fungal Scan (quantifies fungal and “fungal-like” microbes) for targeted fungal community analysis
- HH Beneficials I & II under the Pathogen Scan umbrella to monitor beneficial bacterial and fungal taxa that can help suppress pathogenic biofilms
These sequencing-based services enable you to link shifts in species richness or the emergence of key taxa to biofilm stability, resilience, and function.
Conclusions and Future Directions
Biofilm microbial ecology in Canadian greenhouse systems represents a complex and dynamic field requiring integrated approaches for effective management. The unique environmental conditions of controlled environment agriculture create specific challenges and opportunities for biofilm formation, with significant implications for crop health, system efficiency, and food safety. Understanding the multifactorial nature of biofilm development, including temperature, pH, nutrient availability, and microbial community interactions, is essential for developing targeted management strategies.
Future research directions should focus on developing Canadian-specific management protocols that account for regional climate conditions, energy costs, and crop production systems. The integration of beneficial biofilm communities for plant growth promotion while preventing pathogenic biofilm establishment represents a promising avenue for sustainable greenhouse production. Advanced analytical techniques will continue to enhance our understanding of biofilm ecology, enabling more precise monitoring and management approaches for optimizing greenhouse system performance.
References
Agriculture and Agri-Food Canada. (2021, June 25). What is hydroponics? Government of Canada. https://agriculture.canada.ca/en/science/story-agricultural-science/scientific-achievements-agriculture/what-hydroponics
Al-Daoud, F., & McCreary, C. (2020, September 9). Nutrient solution analysis projects for greenhouses and vertical farms in Ontario (flowers, vegetables and cannabis). ONfloriculture. https://onfloriculture.com/2020/09/09/nutrient-solution-analysis-projects-for-greenhouses-and-vertical-farms-in-ontario-flowers-vegetables-and-cannabis/
Ansari, M. A., Kumar, M., & Patel, A. K. (2022). Optimization of biofilm formation: Influence of temperature, pH and incubation time in a factorial experimental framework. International Journal of Scientific Research in Engineering and Management, 6(11), 1–6. https://ijsrem.com/download/optimization-of-biofilm-formation-influence-of-temperature-ph-and-incubation-time-in-a-factorial-experimental-framework/
Bambara, J., Athienitis, A. K., & Eicker, U. (2021). Decarbonizing local mobility and greenhouse agriculture through residential building energy upgrades: A case study for Québec. Energies, 14(20), 6820. https://doi.org/10.3390/en14206820
Beitelshees, M., Hill, A., Jones, C. H., & Pfeifer, B. A. (2018). Phenotypic variation during biofilm formation: Implications for anti-biofilm therapeutic design. Materials, 11(7), 1086. https://doi.org/10.3390/ma11071086
Billaud, M., Seneca, F. O., Tambutté, É., & Czerucka, D. (2022). An increase of seawater temperature upregulates the expression of Vibrio parahaemolyticus virulence factors implicated in adhesion and biofilm formation. Frontiers in Microbiology, 13, 840628. https://doi.org/10.3389/fmicb.2022.840628
Brackman, S. (2019, March 25). Clean up your act: Sanitation in hydroponic growing. BioSafe Systems. https://biosafesystems.com/news/clean-up-your-act-sanitation-in-hydroponic-growing/
Cabrera Garcia, J. (2019). Identifying clogging factors and thresholds of greenhouse irrigation pipes (Doctoral dissertation, University of Connecticut). UConn Digital Commons. https://digitalcommons.lib.uconn.edu/dissertations/2140/
Chepkwony, N. K., & Brun, Y. V. (2021). A polysaccharide deacetylase enhances bacterial adhesion in high-ionic-strength environments. iScience, 24(9), 103071. https://doi.org/10.1016/j.isci.2021.103071
Chen, Y., Wang, X., Wang, X., Cheng, T., Fu, K., Qin, Z., & Feng, K. (2022). Biofilm structural and functional features on microplastic surfaces in greenhouse agricultural soil. Sustainability, 14(12), 7024. https://doi.org/10.3390/su14127024
Chowdhury, M., Samarakoon, U. C., & Altland, J. E. (2024). Evaluation of hydroponic systems for organic lettuce production in controlled environment. Frontiers in Plant Science, 15, 1401089. https://doi.org/10.3389/fpls.2024.1401089
Culioli, G., Portas, A., Carriot, N., Barry-Martinet, R., & Ortalo-Magné, A. (2024). Impact of phosphate concentration on the metabolome of biofilms of the marine bacterium Pseudoalteromonas lipolytica. Environmental Microbiome, 19, 17. https://doi.org/10.1186/s40793-024-00647-5
Dramm Corporation. (2012). Biofilm in the greenhouse [White paper]. Dramm Corporation. https://www.dramm.com/media/Biofilm%20in%20the%20GreenhouseSM.pdf
Legein, M., Smets, W., Wuyts, K., Bosmans, L., Samson, R., & Lebeer, S. (2022). The greenhouse phyllosphere microbiome and associations with introduced bumblebees and predatory mites. Microbiology Spectrum, 10(4), e01755-22. https://doi.org/10.1128/spectrum.01755-22
Lee, S., Ge, C., Bohrerova, Z., Grewal, P. S., & Lee, J. (2015). Enhancing plant productivity while suppressing biofilm growth in a windowfarm system using beneficial bacteria and ultraviolet irradiation. Canadian Journal of Microbiology, 61(7), 457–466. https://doi.org/10.1139/cjm-2015-0024
Lekshmi, K., & Sreekumar, S. (2025). Optimizing hydroponic nutrient solutions for enhanced growth, root biomass and essential oil yield in the endemic medicinal herb (Plectranthus vettiveroides). Journal of Advances in Biology & Biotechnology, 28(5), 151–166. https://doi.org/10.9734/jabb/2025/v28i52279
Lewandowski, Z., & Beyenal, H. (2009). Methods for imaging and quantifying the structure of biofilms in food processing and other environments. In P. M. Fratamico, B. A. Annous, & N. W. Gunther (Eds.), Biofilms in the food and beverage industries (pp. 99–130). Woodhead Publishing. https://doi.org/10.1533/9781845697167.1.99
Lobo-Cabrera, F. J., del Río Herrero, M., Govantes, F., & Cuetos, A. (2024). Computer simulation study of nutrient-driven bacterial biofilm stratification. Journal of the Royal Society Interface, 21(215), 20230618. https://doi.org/10.1098/rsif.2023.0618
Logozzo, L. A., Soued, C., Bortolotti, L. E., Badiou, P., Kowal, P., Page, B., & Bogard, M. J. (2025). Agricultural land use impacts aquatic greenhouse gas emissions from wetlands in the Canadian Prairie Pothole Region. Global Biogeochemical Cycles, 39, e2024GB008209. https://doi.org/10.1029/2024GB008209
Morales Moreira, Z., & Haney, C. H. (2024). Plant genetic regulation of the microbiome and applications for Canadian agriculture. Canadian Journal of Plant Pathology, 46(5), 546–553. https://doi.org/10.1080/07060661.2024.2340483
National Institute of Food and Agriculture. (2023, March 15). Biological approaches to mitigate biofilm-associated food safety risks in indoor leafy greens production. United States Department of Agriculture. https://portal.nifa.usda.gov/web/crisprojectpages/1029776-biological-approaches-to-mitigate-biofilm-associated-food-safety-risks-in-indoor-leafy-greens-production.html
Rajcoomar, S., Amoah, I. D., Abunama, T., Kumari, S., & Bux, F. (2024). Biofilm formation on microplastics in wastewater: Insights into factors, diversity and inactivation strategies. International Journal of Environmental Science and Technology, 21, 4429–4444. https://doi.org/10.1007/s13762-023-05266-0
Sánchez, O., Ferrera, I., Garrido, L., Gómez-Ramos, M. del M., Rodríguez Fernández-Alba, A., & Mas, J. (2014). Prevalence of potentially thermophilic microorganisms in biofilms from greenhouse-enclosed drip irrigation systems. Archives of Microbiology, 196, 219–226. https://doi.org/10.1007/s00203-014-0957-3
Silva, V., Pereira, J. E., Maltez, L., Poeta, P., & Igrejas, G. (2022). Influence of environmental factors on biofilm formation of Staphylococci isolated from wastewater and surface water. Pathogens, 11(10), 1069. https://doi.org/10.3390/pathogens11101069
Strack, M., Bona, K. A., & Liang, C. (2024). A first assessment of greenhouse gas emissions from agricultural peatlands in Canada: Evaluation of climate change mitigation potential. Wiley Interdisciplinary Reviews: Climate Change, 15(3), e925. https://doi.org/10.1002/wcc.925
Tavano, C. L., Schreiber, F., & Ackermann, M. (2018). Quantifying the formation of viable but nonculturable cells in biofilms. Journal of Visualized Experiments, 133, e57322. https://doi.org/10.3791/57322
Tham, C. A. T., Zwe, Y. H., Ten, M. M. Z., Ng, G. S. Y., Toh, J. Y. L., Poh, B. L., Zhou, W., & Li, D. (2024). Sanitization of hydroponic farming facilities in Singapore: What, why, and how. Applied and Environmental Microbiology, 90, e00672-24. https://doi.org/10.1128/aem.00672-24
Thomas, B. O., Lechner, S. L., Ross, H. C., Joris, B. R., Glick, B. R., & Stegelmeier, A. A. (2024). Friends and foes: Bacteria of the hydroponic plant microbiome. Plants, 13(21), 3069. https://doi.org/10.3390/plants13213069
Thomas, R. R., Kumar, R., Rana, S., Shubhashish, S., Kumar, S., Singh, K., Anjali, & Kaushal, S. (2025). Hydroponic systems: Best practices for biosecurity, disinfection and pest management. Journal of Scientific Research and Reports, 31(4), 306–315. https://doi.org/10.9734/jsrr/2025/v31i42951
Velez Correa, K. E., Leighton, R. E., Decho, A. W., Pinckney, J. L., & Norman, R. S. (2023). Modeling pH and temperature effects as climatic hazards in Vibrio vulnificus and Vibrio parahaemolyticus planktonic growth and biofilm formation. GeoHealth, 7(4), e2022GH000769. https://doi.org/10.1029/2022GH000769
Wang, S., Zhi, L., Shan, W., Lu, H., Xu, Q., & Li, J. (2020). Correlation of extracellular polymeric substances and microbial community structure in denitrification biofilm exposed to adverse conditions. Microbial Biotechnology, 13(6), 1889–1903. https://doi.org/10.1111/1751-7915.13633
West, J., Huber, A., & Carlow, C. (2018, April 9; updated March 2022). Water treatment guide for greenhouses & nurseries [Guidance document]. Flowers Canada (Ontario) Inc. https://www.flowerscanadagrowers.com/uploads/2022/09/guidance%20document%20hts%20project%202018%20low%20res%20final%20revised%2020222.pdf
Wong, L. L., Lu, Y., Ho, J. C. S., Mugunthan, S., Law, Y., Conway, P., Kjelleberg, S., & Seviour, T. (2023). Surface-layer protein is a public-good matrix exopolymer for microbial community organisation in environmental anammox biofilms. The ISME Journal, 17(6), 803–812. https://doi.org/10.1038/s41396-023-01388-y
Yin, W., Wang, Y., Liu, L., & He, J. (2019). Biofilms: The microbial “protective clothing” in extreme environments. International Journal of Molecular Sciences, 20(14), 3423. https://doi.org/10.3390/ijms20143423
Sahib, M. R., Pervaiz, Z. H., Williams, M. A., Saleem, M., & DeBolt, S. (2020). Rhizobacterial species richness improves sorghum growth and soil nutrient synergism in a nutrient-poor greenhouse soil. Scientific Reports, 10, 15454. https://doi.org/10.1038/s41598-020-72516-3
Disclaimer
The information presented in this blog is based on collating published peer-reviewed scientific literature and sources that we consider reliable. This is by no means an exhaustive review of pathogens and IPM strategies. This blog provides a brief overview of what is known about pathogens. We encourage growers to do more research on the pathogens concerning their crops and hydroponic systems. We are not plant pathologists; therefore, the information presented should not be used as professional advice for treating pathogens or operating your system.